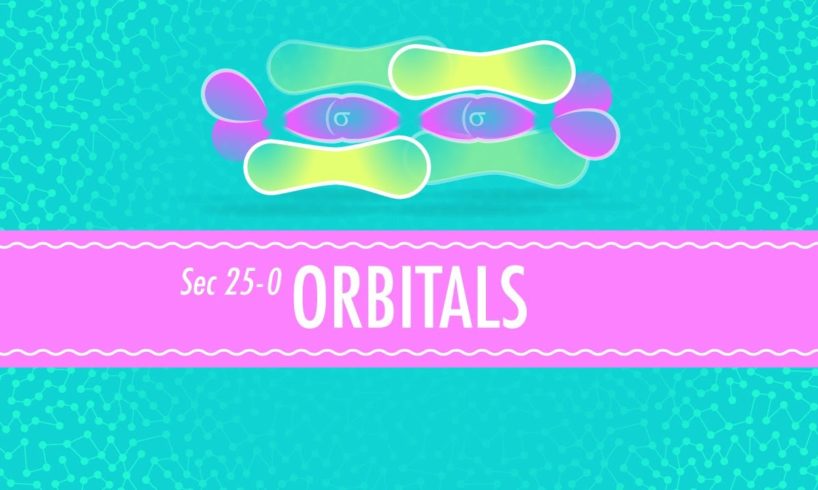
In this episode of Crash Course Chemistry, Hank discusses what molecules actually look like and why, some quantum-mechanical three-dimensional wave functions are explored, he touches on hybridization, and delves into sigma and pi bonds.
Pssst… we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: https://apple.co/3d4eyZo
Download it here for Android Devices: https://bit.ly/2SrDulJ
—
Table of Contents
Molecules: Clumpy Globs… 0:18
Quantum-Mechanical Three-Dimensional Wave Functions 3:06
S & P Orbital Hybridization 5:27
Sigma & Pi Bonds 7:32
Hybridized Orbitals 5:52
Crash Course is on Patreon! You can support us directly by signing up at http://www.patreon.com/crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook – http://www.facebook.com/YouTubeCrashCourse
Twitter – http://www.twitter.com/TheCrashCourse
Instagram – https://www.instagram.com/thecrashcourse/
CC Kids: http://www.youtube.com/crashcoursekids
source






Pssst… we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: https://apple.co/3d4eyZo
Download it here for Android Devices: https://bit.ly/2SrDulJ
9:06
You're porygon just evolved into porygon Z
Thank you for such wonderful information 🔥
What
Hank Green you have saved my grades more than once. Not all heros wear capes.
yup, trying to pass a test. thank you for this video. helped me with my reading. those darn books expect me to know what theyre saying in just a paragraph.
we actually learn the s,p,d and f orbitals as k,m,n,l in India (as far as i know)
Woah woah woah slow down buddy
Hank you frank you 😁😁
Yes, I'm just trying to pass a test. (Thanks for the video, I mean it, really~!)
Someone tell my A-levels chemistry teacher the drawing everything on whiteboard does not work at all please~ XD
My brain after watching roughly 7 times:
🤪🤪🤪 greek wut
If you made it past high school it gets interesting at (5:10).
Wow
So amazing
I just love the way they talk
Keep it up 👍👍👍👍
This guy looks and talks like James Potter. Never fails to remind me of him.
I had to check if the video speed was accelerated
Wait. How do two s orbitals and three p orbitals combine to make four sp orbitals
😭😭😭
That was very informative… loved the dsp2 hybrid look, TFS!
Surely at 4:49 the 2Pz and 2Py orbitals are mixed up???
2:28 hydllager